Fundamentals of Adhesion
Selecting the proper adhesives for a nameplate, label or membrane switch application requires consideration of environmental, surface, appearance and other performance requirements. Our purpose here is to cover some of the principles of adhesion.
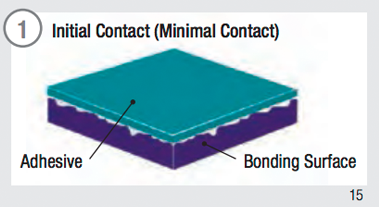
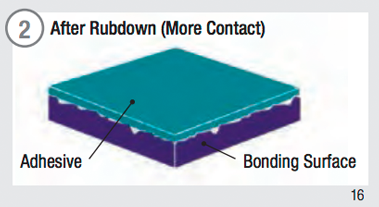
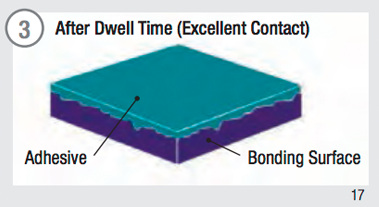
Surface contact is fundamental to adhesive performance. To maximize adhesive contact on a surface:
- It must be dry and free of contaminates.
- Firm pressure must be applied to increase the flow and contact of the adhesive with the substrate.
- Time and temperature will increase the surface contact and adhesion values.
- Oil contaminated materials may be addressed with the 3M™ Adhesive 300LSE or 350 families.
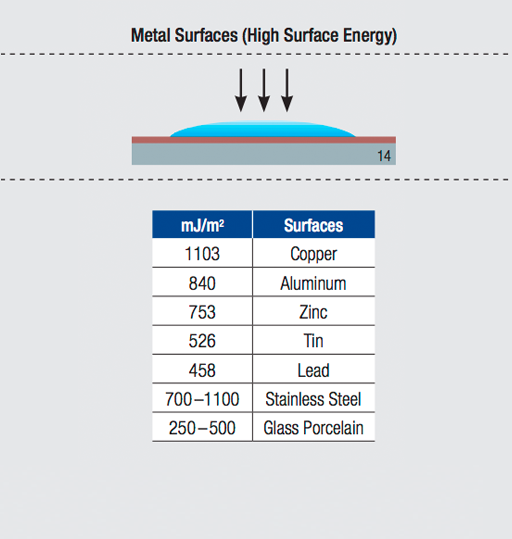
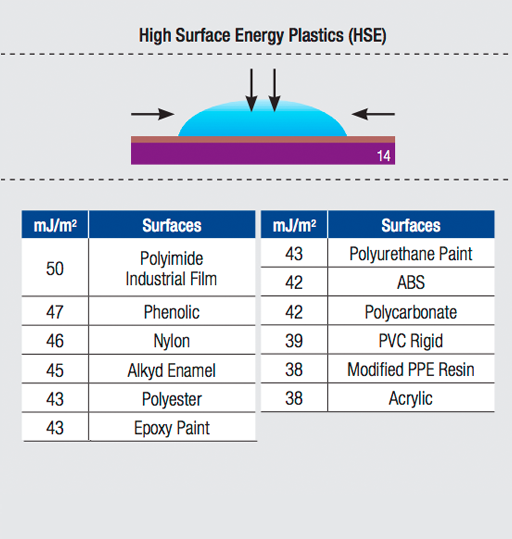
Adhesion is the molecular force of attraction between unlike materials. The strength of attraction is determined by the surface energy of the material. The higher the surface energy, the greater the molecular attraction. The lower the surface energy, the weaker the attractive forces.
Greater molecular attraction results in increased contact between an adhesive and substrate. In other words, for a high surface energy material, the adhesive can flow (or “wet-out”) to assure a stronger bond.
Consider an automobile that has not been waxed for a long time. When water contacts the surface, it spreads in large puddles. The unwaxed car surface exhibits high surface energy — the molecular attraction allows the water to flow.
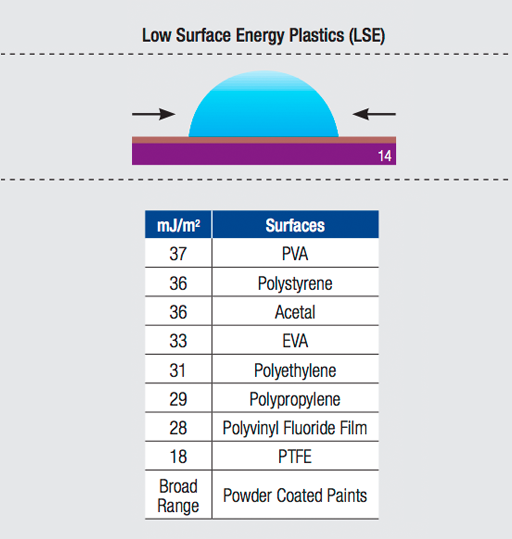
In comparison, water beads up into small spheres on a freshly waxed car. It is an example of low surface energy — the liquid (or adhesive) does not flow out.
Surface energy is measured by dynes per centimeter. The dyne level is the actual reading of the critical surface tension.
Modified acrylic and synthetic adhesives with better flow (or "wet-out") characteristics have been developed to adhere to low surface energy substrates. The Surface Energy Chart below compares the relative surface energy of commonly used substrates.
3M™ High Performance Acrylic Adhesive 200MP will not readily adhere to substrates categorized as having "low surface energy." However, 3M acrylic adhesives have been designed to adhere to low surface energy plastics, and should be considered for those applications.
Surface Energy Chart
Adhesive Surface Contact